Summary
IgG4-related disease (IgG4-RD) is a systemic fibro-inflammatory disorder that affects multiple organs, including the salivary and lacrimal glands, pancreas, and kidneys, manifesting through symptoms like gland enlargement, autoimmune pancreatitis, and retroperitoneal fibrosis. The disease is marked by elevated serum IgG4 levels, infiltration of IgG4-positive plasma cells, and storiform fibrosis in affected tissues. Currently, no drug is approved specifically for treating IgG4-RD. Hyperactivation of plasmacytoid dendritic cells (pDCs), the main cellular source of interferin-a (IFN-a) and interleukin-33 (IL-33) , is the driving cause of the disease, though the imbalance of Treg/Teff also play a role in the disease pathogenesis. The target of IPG007, a novel GPCR, is predominantly expressed on pDCs and Treg cells, and plays a crucial role in their activation and migration. IPG007 potently blocks the target-mediated pDC and Treg migration in vitro, and dramatically inhibits pDC infiltration in the lesion sites, resulting complete inflammation resolution and eradication of tissue fibrosis in a Poly (I:C)-induced MRL/MpJ mouse model, which mirrors human IgG4-RD.
Phase I clinical trial of IPG007 in health subjects has demonstrated favorable safety and pharmacokinetic profile. With the successful completion of 6/9-month long-term toxicology study showing excellent safety and tolerability, Phase II clinical trial on IgG4-RD is about to be initiated soon. Thus, IPG007 is a typical disease modifying asset, with significant advantage over the traditional therapies.
Mechanism of Action
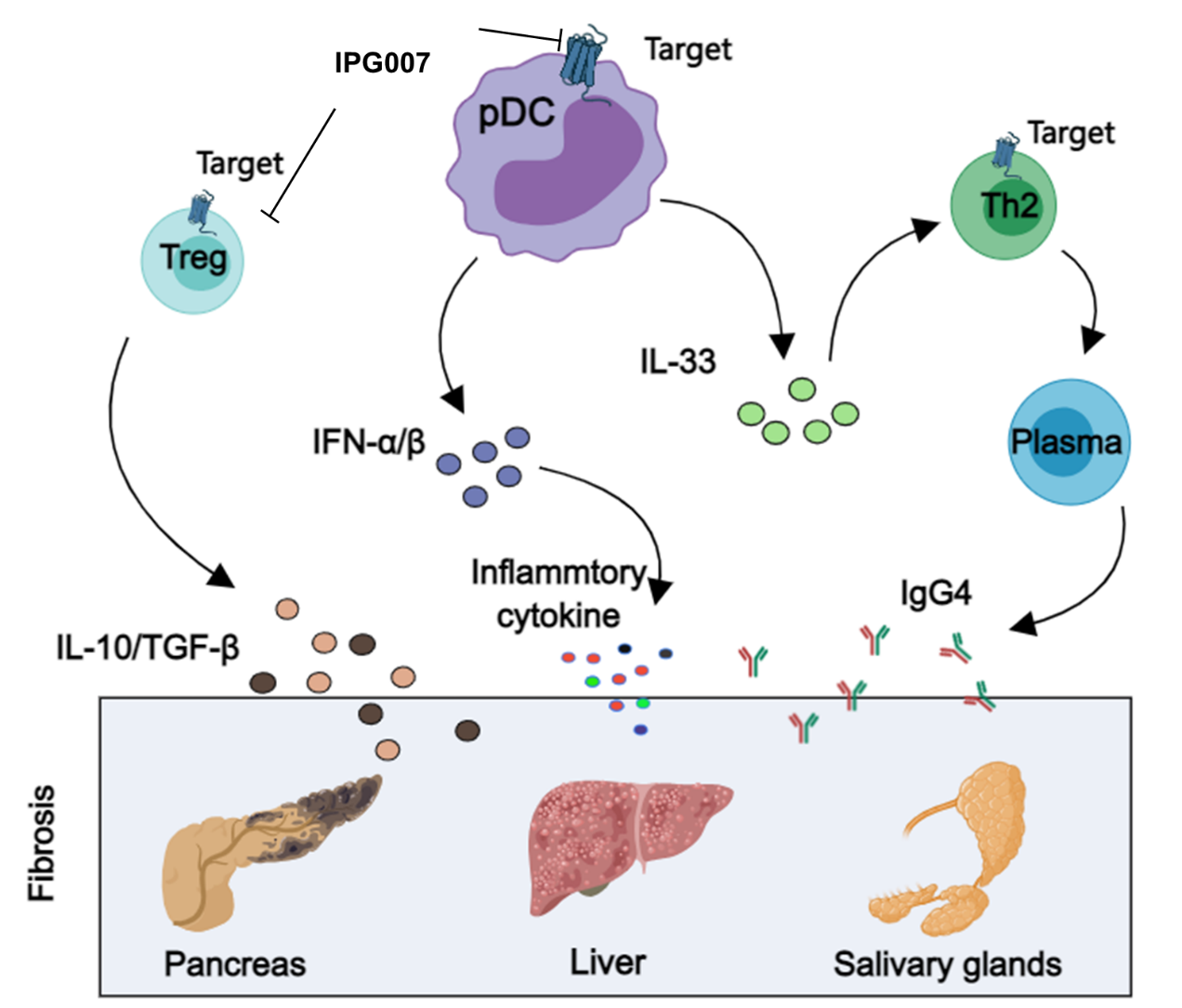
■ The GPCR target is expressed on key immune cells involved in the pathogenesis of IgG4-RD, including Treg cells, pDCs, and Th2 cells.
■ Following pDC activation, high levels of IFN-α and IL-33 are produced. IFN-α stimulates the release of various inflammatory cytokines, while IL-33 enhances the production of Th2 cytokines (such as IL-4) and influences plasma cell activity, leading to inflammation and fibrosis in glands affected by IgG4-RD.
■ The IPG007 inhibits pDC migration to the lesion sites, downregulates IFN-α and IL-33, both of which are central to fibrosis and B cell activation, thereby facilitating inflammation resolution and blocking fibrosis progression.
Key Differentiation
■ The Target of IPG007 plays a key role in the migration of hyperactivated pDCs, the main cellular source of INF-a and IL-33 that induce B cell activation and fibrosis, respectively. Thus, IPG007 is a disease-modifying agent that specifically eradicates IgG4-RD associated pathologies. IPG007 differentiates itself from B cell-depletion therapies, such as anti-CD19/CD20 antibodies, which nonspecifically deplete B cells, posing the risk to persistent hypogammaglobulinaemia and life-threatening infection.
■ Unlike glucocorticoids which broadly suppress immune system and cause several side effects, IPG007 specifically inhibits the hyperactivation and migration of pDCs, resulting in complete blockade of fibroinflammatory process, with no remarkable drug-related adverse effects as evidenced by phase I clinical trial.
In vivo Properties
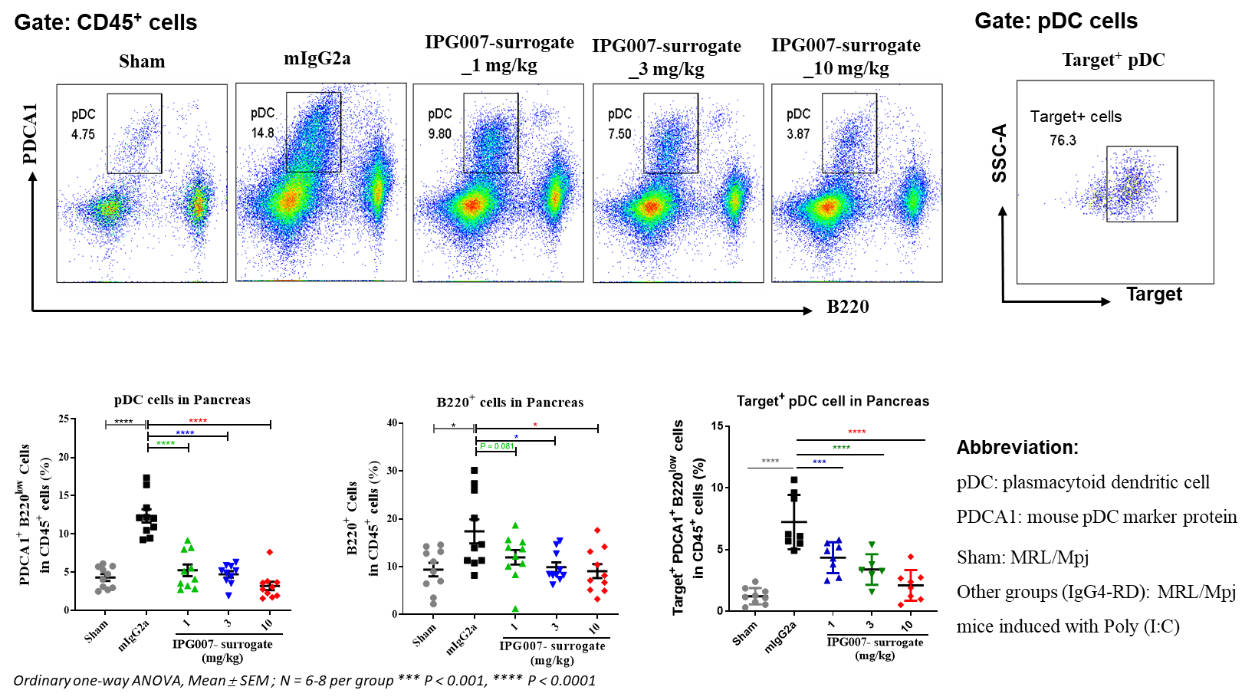
■ As IPG007 specifically antagonizes human target GPCR signaling, without affecting murine target GPCR, a murine antagonist of the target GPCR with equivalent potency (IPG007-surrogate) was used for the pre-clinical studies. IPG007-surrogate dose-dependently reduced pDCs and B cells infiltrated in the pancreatic tissues of IgG4-related autoimmune pancreatitis (type I AIP) model.
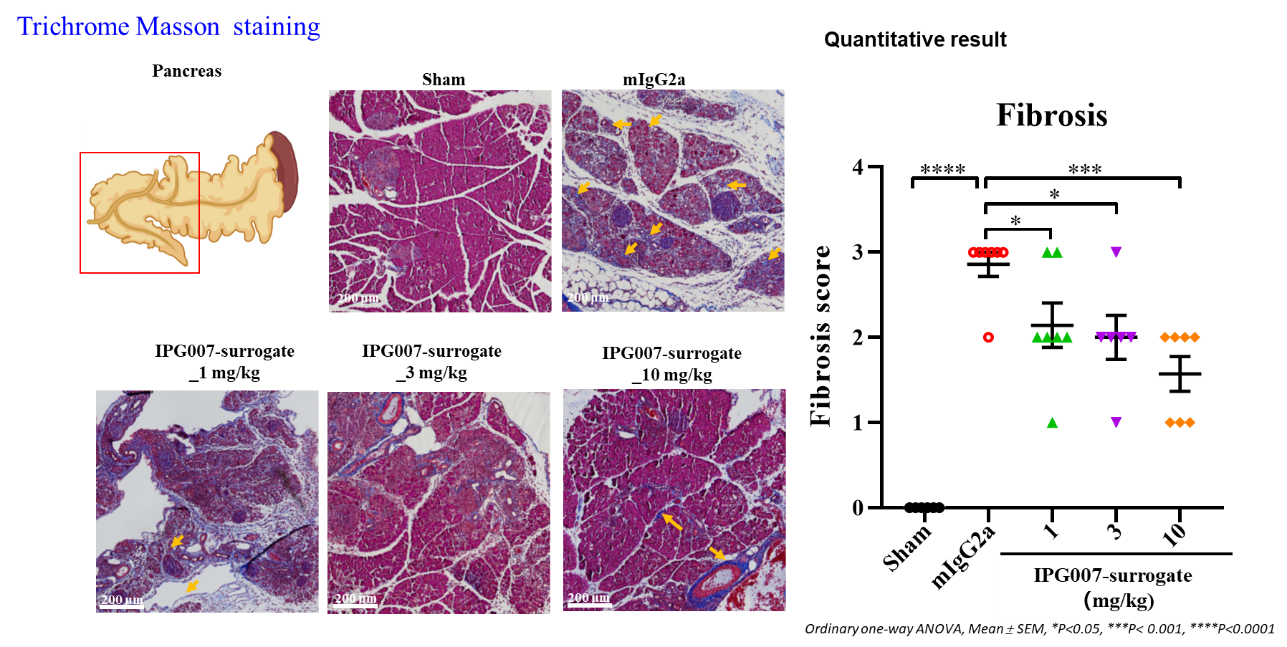
■ IPG007-surrogate dose-dependently reduced fibrosis in IgG4-related autoimmune pancreatitis (type I AIP) model.
Clinical trials
■ Phase I clinical trials demonstrated no severe adverse events (> grade 2) during the dose-escalation phase, with a clear linear dose-exposure relationship.
■ With the IND approval and successful completion of 6/9-month long term toxicology studies, a Phase II clinical trial for IgG4-RD is about to be initiated soon.
Cpyright © 2023 Nanjing Immunophage Biotech Co.,Ltd All Rights Reserved.
